The human endothelium forms a permeable barrier between the blood stream and the surrounding tissue, strictly regulating the passage of immune cells and metabolites. For its correct function, regulation of cell-cell contact dynamics between endothelial cells is essential. In this context, we focus particularly on the adherens junctions system, which is predominantly composed of intercellular adhesion proteins such as VE-cadherin and nectin, as well as their associated proteins.
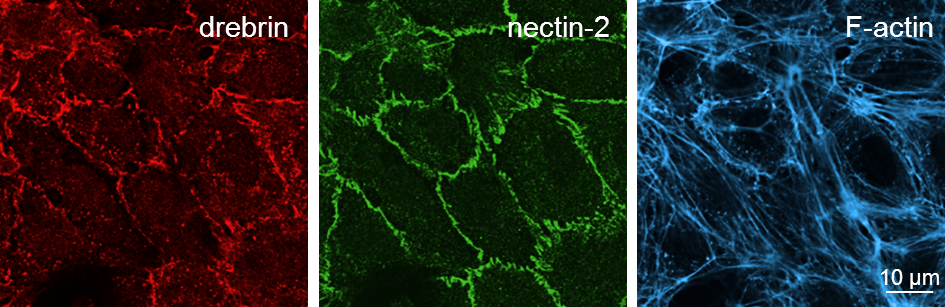 Drebrin and nectin-2 are localized at endothelial cell-cell junctions
Confocal images of HUVEC with immunofluorescence staining of drebrin (left), nectin-2 (middle) and F-actin (right). Click image to enlarge. |
Drebrin, an F-actin binding protein has been included in the growing list of “junction associated” proteins, but its potential role in adherens junction dynamics has been unclear so far. We could show that knockdown of drebrin leads to functional impairments of endothelial monolayers, shown by rupturing of HUVEC monolayers cultured under constant unidirectional flow conditions, which mimic blood flow in vessels.
|
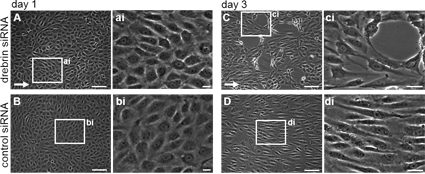 |
Drebrin knockdown monolayers show ruptures while cultured under constant flow
Images of HUVEC monolayers, treated with drebrin-specific (A,C) or control siRNA (B,D), and seeded in microslides. Cells were submitted to constant fluid shear stress for 1 - 3 days. The enlarged images (ai-di) show boxed regions of (A-D). Arrows indicate the direction of flow. Note the rupture of drebrin knockdown monolayers at day 3. Bars, 10 µm in (Ai-Di), 100 µm in (A-D). (Rehm et al. JCS, 2013) Click image to enlarge. |
The observed weakening of cell-cell contacts is characterized by a specific and complete loss of nectin from adherens junctions, due to its endocytosis and subsequent degradation in lysosomes.
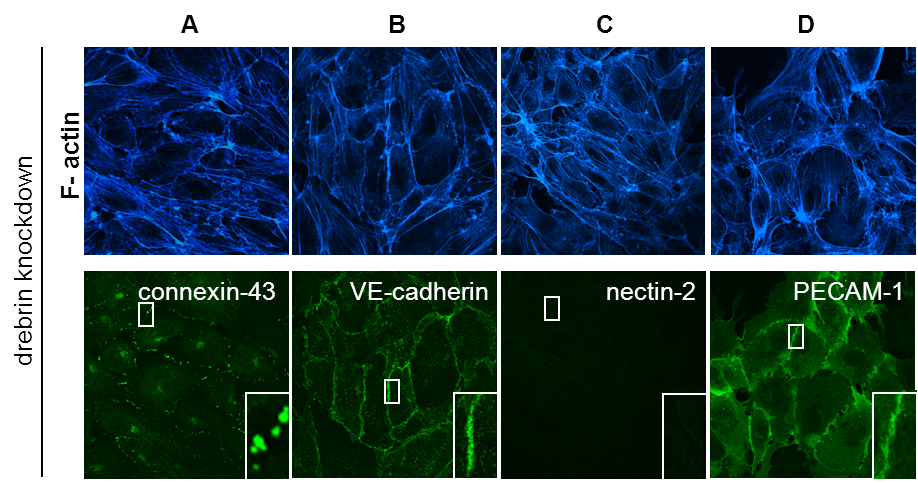
Drebrin knockdown leads to loss of nectin-2 from cell-cell junctions
(A-D) Confocal micrographs of HUVEC transfected with a pool of 4 drebrin-specific siRNAs. Monolayers were stained for F-actin (A-E), connexin-43 (A), VE-cadherin (B), nectin-2 (C), PECAM-1 (D). Note specific loss of nectin-2 from cell-cell junctions in drebrin knockdown cells. Bars, 10 µm. (Rehm et al. JCS, 2013) Click image to enlarge. |
|
Performing co-immunoprecipitation and GST-pulldown experiments, we could show that drebrin does not interact with nectin-2 directly, but with its binding partner, afadin. This was further confirmed by mitochondrial retargeting experiments, where drebrin’s afadin-binding polyproline region fused to a mitochondrial targeting signal is sufficient to relocalize afadin towards the outer membrane of mitochondria.
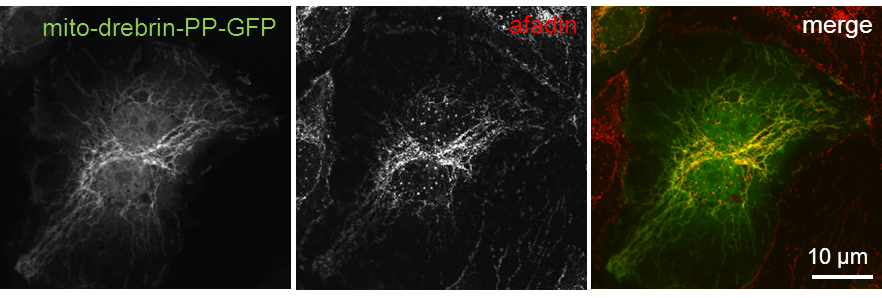
Drebrin’s polyproline region binds afadin and relocalizes it to mitochondria
Confocal micrographs of HUVEC expressing mito-drebrin-PP-GFP (left). Cells were fixed and immunostained for afadin (middle). Bars, 10 µm. (Rehm et al. JCS, 2013) Click image to enlarge. |
|
Furthermore, we could demonstrate that drebrin’s role in maintaining junctional integrity is to link the nectin/afadin system to the cortical F-actin network. Evidence is provided by expressing minimal rescue constructs containing exclusively afadin´s PDZ region (binding nectin) coupled to drebrin´s F-actin binding region, which restores junctional nectin under knockdown of both drebrin and afadin.
|
|
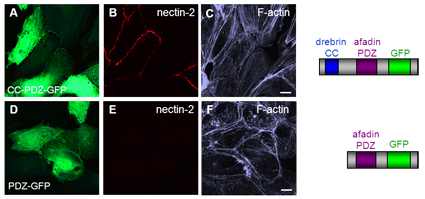
Recovery of nectin at cell-cell junctions by minimal rescue constructs
HUVEC treated with drebrin and afadin siRNA for 3 days coexpressing the CC-PDZ-GFP construct (A) binding to nectin-2 and F-actin, or GFP as a control (D). Confocal micrographs of HUVEC monolayers stained for nectin-2. Expressed domain constructs are schematically depicted on the right side. Bars, 10 µm. (Rehm et al. JCS, 2013) Click image to enlarge. |
These results contribute to our current understanding of how junctions are regulated in the endothelium under vascular flow. In particular, the newly identified interaction between drebrin and afadin is shown to be crucial for nectin-based junctional integrity. They also indicate that, contrary to previous assumptions, nectins are not only important for formation of cell-cell junctions but also for their upkeep. Current work concerns the dynamic regulation of the F-actin-drebrin-afadin-nectin chain in maintaining endothelial integrity.
K. Rehm et al. J. Cell Sci., 2013